2.3. Types of Intermolecular Forces
Parts of molecules can influence each other through means other than direct covalent bonds. When these noncovalent interactions occur between two molecules, they are known as intermolecular forces. The types and strengths of noncovalent interactions a molecule is capable of exerting are a direct result of the structure itself.
All intermolecular forces are attractive forces between parts of the molecules. In all cases they arise from local charge interactions: areas that have an excess of electron density are attracted to areas that have a deficiency in electron density.
2.3.1. Electrostatic Interactions – Interactions of Ionic Charges
Electrostatic interactions are the simplest case to consider. These arise from the attractive force of full charges (formal charges) between a cation and an anion (Figure 2.31). In most cases these are simply ionic bonds (see Section 1.1.6).
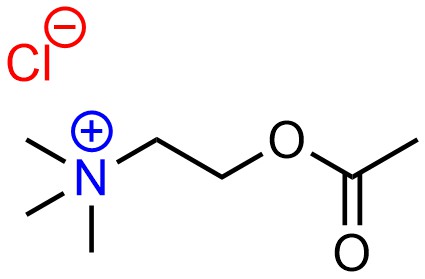
Figure 2.31 – Example of an Electrostatic Interaction.
Because electrostatic interactions arise from full charges they are by far the strongest type of intermolecular force.
2.3.2. Dipole-Dipole Interactions – Interactions of Partial Charges
Dipole-Dipole interactions are the result of a permanent net dipole. These arise from the presence of partial charges between areas that are electron-poor and areas that are electron-rich (Figure 2.32).
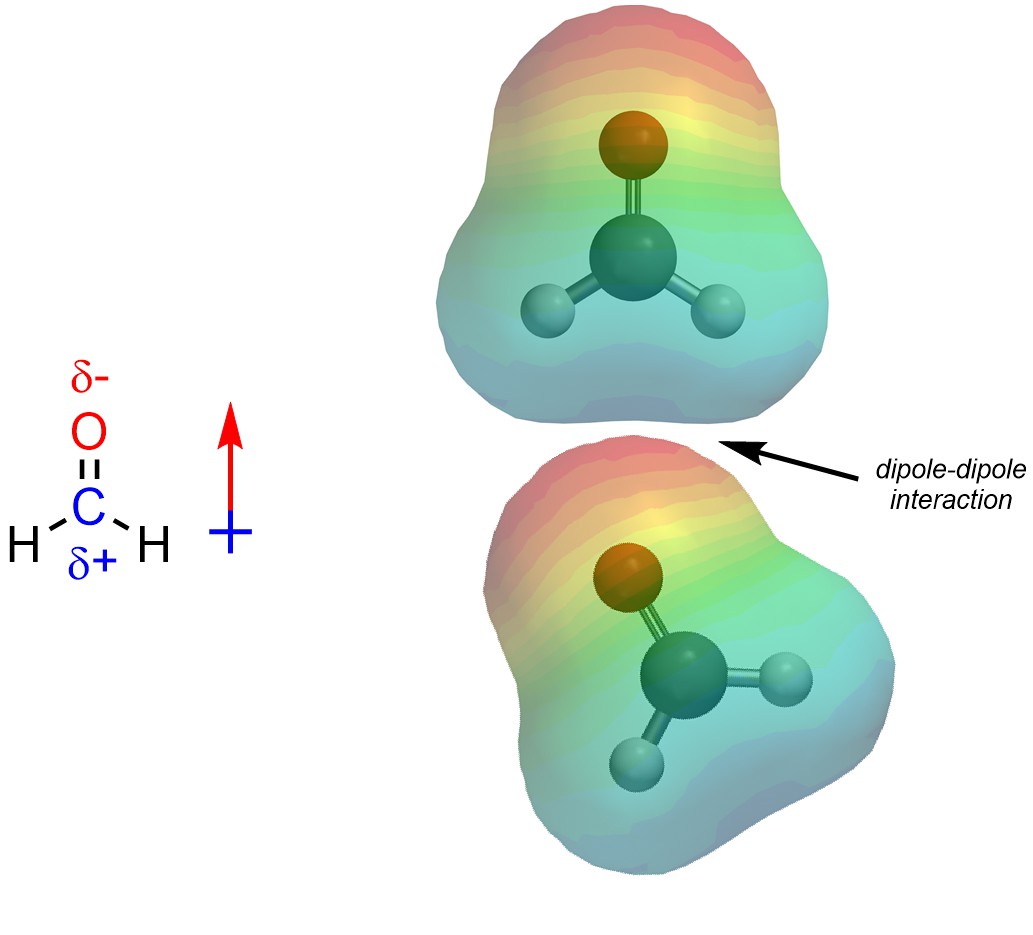
Figure 2.32 – Example of a Dipole-Dipole Interaction.
Because dipole-dipole interactions arise from permanent partial charges they are usually the second strongest type of intermolecular force.
2.3.2.1. Hydrogen Bonding
A certain type of dipole-dipole interaction is so common it has its own name: hydrogen bonding. In hydrogen bonding the partial charges are on a hydrogen that is attached to a heteroatom and another heteroatom (Figure 2.33). Molecules that have a lone pair on an electronegative heteroatom are hydrogen bond acceptors. The most common atoms acting as hydrogen bond acceptors are nitrogen and oxygen. Molecules that have a hydrogen attached to an electronegative heteroatom are hydrogen bond donors. Many molecules, such as water, have both properties and can be donors and/or acceptors.
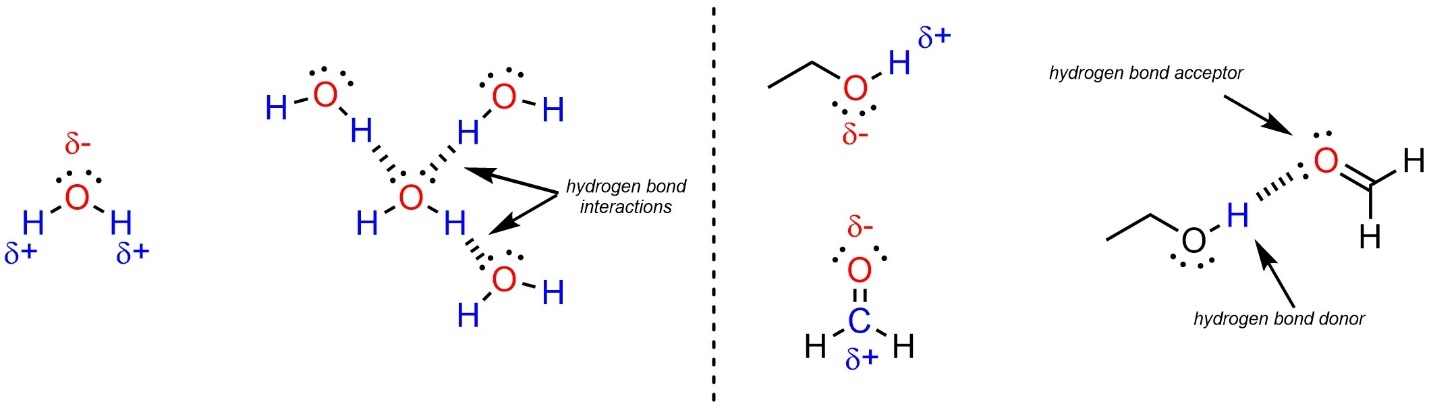
Figure 2.33 – Examples of Hydrogen Bonding Interactions.
It is important to remember that hydrogen bonding is a type of dipole-dipole interaction. As such it requires partial charges on the atoms involved. For example, since C-S and S-H bonds are not polar (see Section 2.2.2.11) thioalcohols are not capable of being hydrogen bond donors or acceptors.
Hydrogen bonding has one special characteristic. Since hydrogens can be added and removed easily (acid-base reactions) hydrogen bonds tend to be slightly stronger than other dipole-dipole interactions because there is a small degree of covalent bonding occurring; the hydrogen is being partially deprotonated and can be viewed as being semi-covalently bonded to both heteroatoms. This makes these interactions (slightly) stronger since it takes more energy to break the two parts apart.
2.3.3. Dispersion // London Forces
Dispersion interactions (sometimes called London forces) are the result of a temporarily induced dipole. These interactions are the most difficult to conceptualize. Consider the electron distribution of methane (Figure 2.34). Unlike in molecules with a net dipole, the electron density is fairly evenly distributed around the molecule.
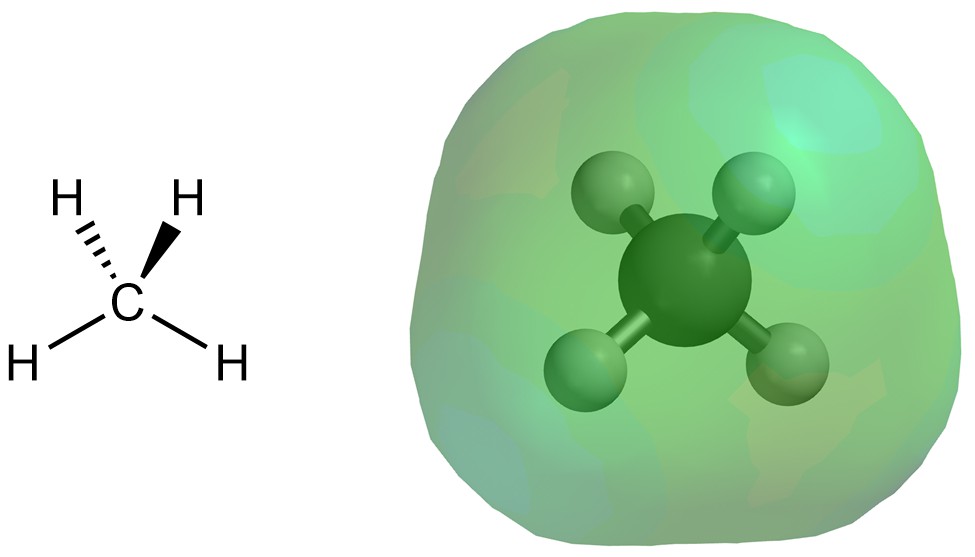
Figure 2.34 – Electron Density Distribution for Methane.
It is important to remember that these electron density clouds represent a probability distribution. That is, this is the area where the electrons can usually be found (>95%) and on a time–averaged scale they will be found at all these places with approximately equal frequency. There are discrete electrons moving around in this area. At any given point in time their distribution is (semi-)random. While on a time-averaged scale there are no electron-rich or electron-poor areas, at specific moments in time there will be. These charges are extremely transient, lasting for tiny fractions of a second. However, during those times they can induce changes to the electron distribution in nearby molecules (Figure 2.35). This can bring small, fleeting partial charges close together for an attractive interaction.
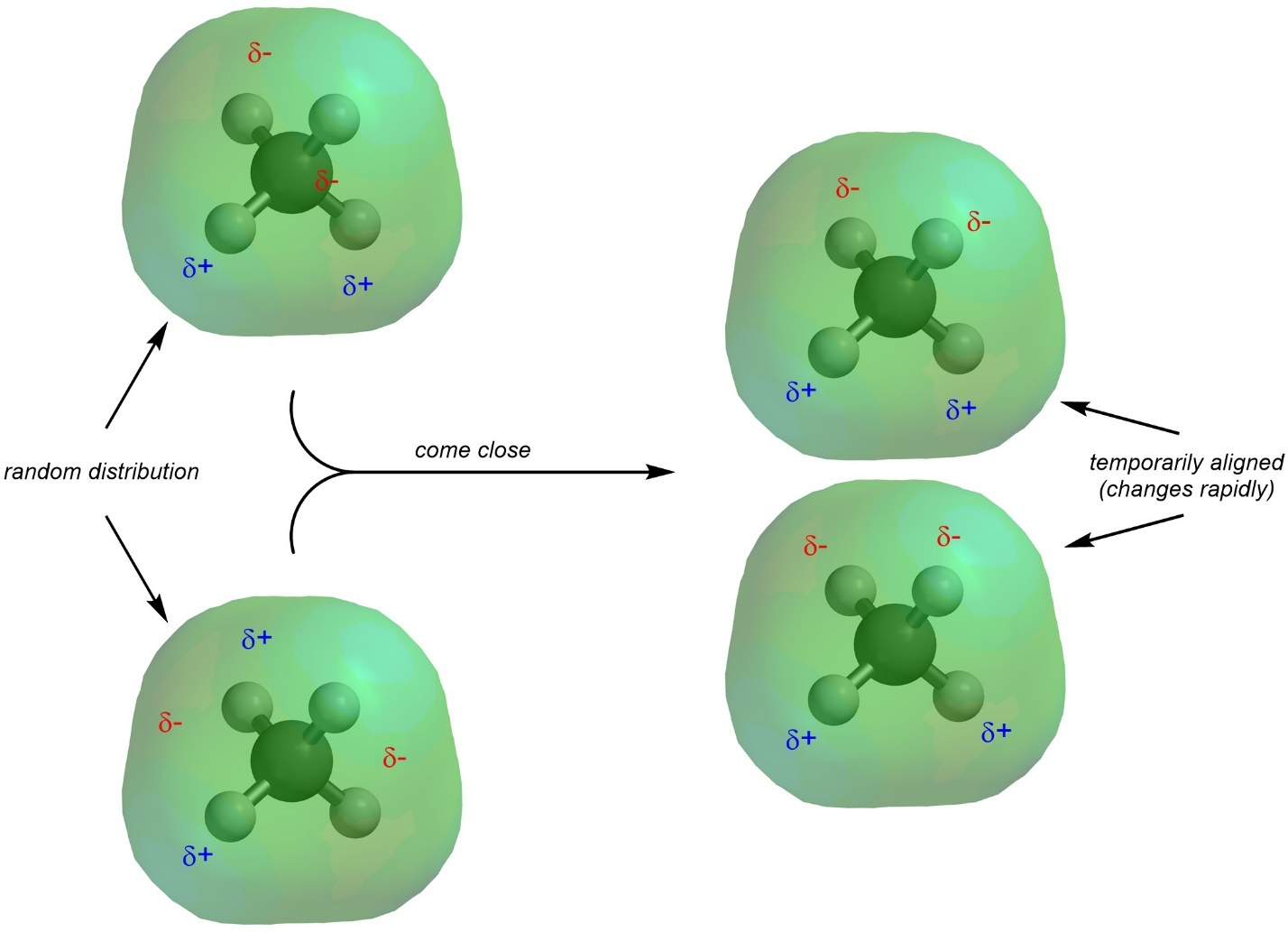
Figure 2.35 – Representation of Temporarily Induced Dipoles for Dispersion Interactions.
Because dispersion interactions arise from temporarily induced partial charges they are by far the weakest type of intermolecular force. Usually dispersion is only relevant if there are large surface areas brought into contact with each other (see Section 2.4).

